HealthPRO Canada News
May 11, 2021
HealthPRO and the Institute for Safe Medication Practices (ISMP) Canada collaborate to prevent potential harm from neuromuscular blocking agent
During HealthPRO’s recent Product Evaluation process for its injectables RFP, they noticed that a paralytic medication – rocuronium – submitted for evaluation was missing some key elements in its packaging that can help prevent medication errors.
Paralytic drugs, also known as neuromuscular blocking agents, bear a heightened risk of causing significant patient harm when they are used in error, and should carry a prominent warning including a red cap and ferrule with lettering that reads: “Paralyzing Agent” or “Warning: Paralyzing Agent” in white.
HealthPRO worked with the supplier, Auro, to outline the packaging changes that would be required. Commendably, Auro is now working closely with Health Canada to have these changes quickly approved.
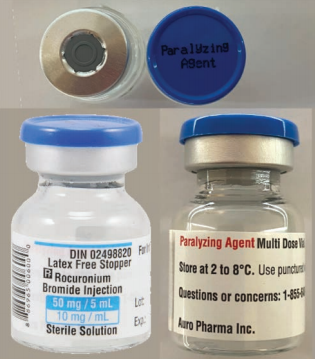
Given that the label changes are still in progress, HealthPRO and the Institute for Safe Medication Practices (ISMP) Canada, have worked together to create a medication alert bulletin alerting frontline healthcare workers of the risk associated with the current stock.
Suppliers of paralytic medications are strongly encouraged to follow the packaging recommendations outlined by the Institute for Safe Medication Practices (ISMP) Canada and Health Canada’s Good Package and Label Practices Guide for Prescription Drugs, especially as they pertain to:
- the ferrule;
- cap colour;
- and warning statement
For more information, please contact Tracy Gallina, Clinical Director at tgallina@healthprocanada.com.


